Acid Base Titration Lab Report Answers
The titration proceeds until the equivalence pointis reached where the number of moles of acid H is equal to the number of moles of base OH -. Acid-base titration can also be used to find percent purity of chemicals.
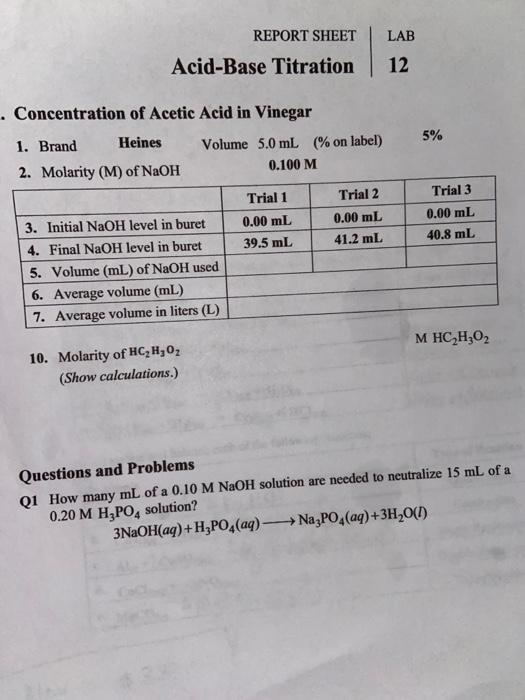
Solved Lab Report Sheet Acid Base Titration 12 Chegg Com
A standardized solution is a solution of known molarity.

. Exterior tile needs to have a cementious type. Questions and Answers 3618. 10mL 001mL of oxalic acid used for each trial 3 drops of indicator were used for each trial Air pressure 10328 0005kPa Temperature 225 o C 005 o C All three trials were successful as the solutions turned a light pink color.
Buffer solution A solution that resists changes in pH even when a strong. What is hypothesis of a acid base titration. Calculate the average value for the baseacid ratio.
Write the completed sentence in the response in your lab report Explain why you can or can NOT. 2 At the end of an acid-base titration the products in the Erlenmeyer flask are always a _____ and _____. You will make a standard solution.
Justify clearly for each answer. Titration with sodium hydroxide and vinegar Constants 10mL 001mL of vinegar used for each trial. Using the molarity of NaOH and amount of base added along with the volume of the H 2 SO 4 solution the concentration of H 2 SO 4 solution can be determined.
If the product contains an acid or base this equation is usually answered by a titration. How waterproof is exterior plywood if used for base to lay tile on an exterior deck. A procedure for making this kind of determination is called an ACID-BASE TITRATION.
Acid Base Titration Questions and Answers Test your understanding with practice problems and step-by-step solutions. Re-fill the burets and repeat the procedure 2 more times for a total of 3 trials. Data analysis 1 390 mols1l 01469l 0057291 mols of naoh 2 moles hclmoles naoh 0057291 mols of hcl 3 0057291 mols hcl01l 57291 m 4 390 mols1l 02553l 0099567 mols of naoh 5 mols of acetic acid mols of naoh 6 0099567 mols hcl01l 99567 m 7 0099567 mols 60gmol597402g 8 105gml 1000ml105x103 g 597402 g.
In the acid-base titration being performed today an H 2 SO 4 sample of unknown concentration will be titrated with a NaOH solution of known concentration. Base and the initial and final volume of extra acid added to this flask 8. When the acid and base react they form NaCl sodium chloride which is also known as table salt.
From these values calculate the base to acid ratio. Acid-Base titrations can be used to measure the concentration of an acid or base in solution to calculate the formula molar mass of an unknown acid or base and to determine the equilibrium constant of a weak acid Ka or of a weak base Kb. In equation 1 the acid is HCl hydrochloric acid and the base is NaOH sodium hydroxide.
Exterior grade plywood is not waterproof and is not meant to be used as a base for tile in exterior applications. Browse through all study tools. These chemicals called primary standards can be used directly to make a solution with a very accurate molarity.
At 25 C the pH of a 500 mL sample of 020 M CH 3 CH. TITRATION OF ACIDS AND BASES Reminder Goggles must be worn at all times in the lab. To determine the concentration of the unknown solution and to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH.
Dont waste time Get Your Custom Essay on Acid Base Titration Lab Report Discussion Get High-quality Paper. Titration curve A plot of pH Vs millilitres of titrant showing the manner in which pH changes Vs millilitres of titrant during an acid-base titration. Some chemicals are very pure and easy to handle.
A Lab Practical Introduction In this experiment you will work with standardized solutions. In the chemistry laboratory it is sometimes necessary to experimentally determine the concentration of an acid solution or a base solution. Got 100COOH is a carboxylic acid that reacts with water according to the equation above.
Equivalence point The point at which just an adequate reagent is added to react completely with a substance. Calculate the total final volume of acid and final volume of base added. When a weak acid reacts with a weak base the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger.
In this procedure a solution of.

Solved Section Titration Lab Report Sheet Table 1 Acid Base Chegg Com

Solved Report Sheet Acid Base Titration Lab 10 M A Chegg Com
![]()
Acid Base Titration Lab Answers Exercises Chemistry Docsity
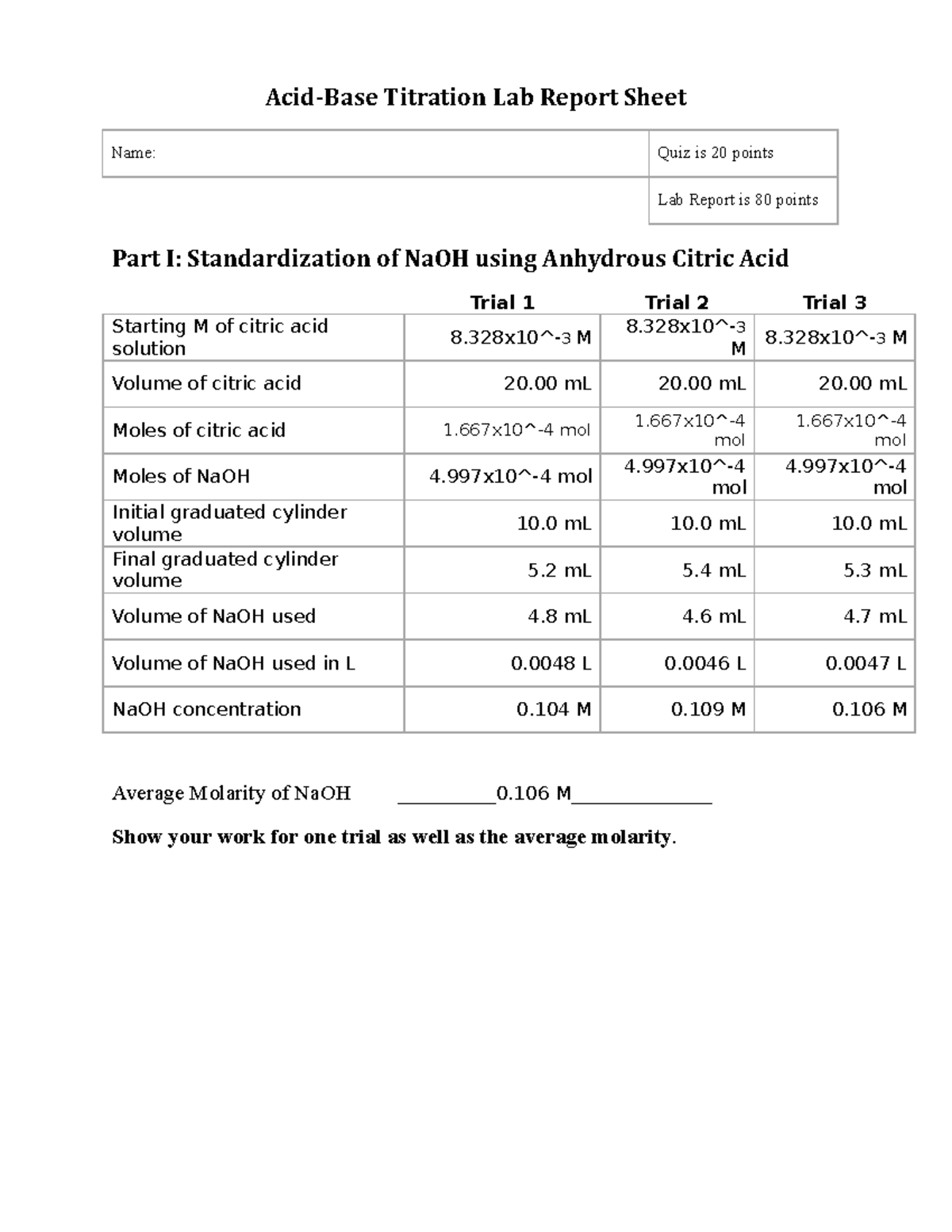
Acid Base Titration Lab Part Ii Determination Of Citric Acid In Unknown Impure Sample Trial 1 Studocu
No comments for "Acid Base Titration Lab Report Answers"
Post a Comment